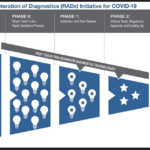
- NIH mobilizes national innovation initiative for COVID-19 diagnostics
- Initiative aims to speed delivery of accurate, easy-to-use, scalable tests to all Americans.
- With a $1.5 billion investment from federal stimulus funding, the newly launched Rapid Acceleration of Diagnostics (RADx) initiative will infuse funding into early innovative technologies to speed development of rapid and widely accessible COVID-19 testing
- NIH is urging all scientists and inventors with a rapid testing technology to compete in a national COVID-19 testing challenge for a share of up to $500 million over all phases of development.
Category Archives: COVID-19 News and Info
Moderna Announces IND Submitted to U.S. FDA for Phase 2 Study of mRNA Vaccine (mRNA-1273) Against Novel Coronavirus
- 600 participant Phase 2 study to begin upon IND acceptance and safety data from ongoing NIH-led Phase 1 study
- Planning underway for Phase 3 study; study expected to begin in the fall of 2020
Sonora Quest Laboratories Now Offering COVID-19 Antibody Testing in Arizona
TEMPE, Ariz. (April 24, 2020) – Sonora Quest Laboratories, the market share leader in diagnostic laboratory testing in Arizona, announced today that it will ramp up its fight against COVID-19 and offer antibody testing for coronavirus (COVID-19) using blood specimens. With this new offering, Sonora Quest now provides healthcare providers in Arizona access to a COVID-19 antibody test as well as the molecular diagnostic testing that has been offered since mid-March.Continue reading
Banner Health urges former COVID-19 patients to donate plasma for current patients
Banner Research provides Donation Coordinators to assist potential donors
PHOENIX (April 24, 2020) – With the need for “convalescent plasma” to treat critically ill COVID-19 patients ramping up as cases rise, Banner Research is providing donation coordinators in metro Phoenix, Tucson and Northern Colorado to help recovered COVID-19 patients get through the plasma donation process. The donation coordinators are equipped to assess qualifying criteria for potential donors, answer questions and explain how the donation process works.Continue reading
Johnson & Johnson Announces Collaboration to Expand Manufacturing Capabilities For its COVID-19 Vaccine Candidate in Support of the Company’s Goal to Supply More Than One Billion Vaccine Doses Globally
Company Signs Agreement with Emergent BioSolutions in the U.S. As Part of its Investment
First in Series of Anticipated Strategic Collaborations Designed to Further the Company’s Goal of Ensuring Global Supply of a Safe and Effective Vaccine for COVID-19Continue reading
Pathogen and Microbiome Institute at NAU to test the antiviral activity of 2X-121, a Danish Company’s PARP inhibitor, against Coronavirus
Hørsholm, Denmark (April 22nd, 2020) – Oncology Venture A/S (“OV” or the Company) today announced that it has entered into an agreement with the Pathogen and Microbiome Institute at Northern Arizona University (NAU) to test the antiviral activity of 2X-121, its PARP inhibitor, against Coronavirus.Continue reading
Novel coronavirus detected, monitored in wastewater
Within weeks of arriving on the world stage, SARS-CoV-2 has managed to encircle the globe, leaving illness, mortality and economic devastation in its vast wake. One of the central challenges facing health authorities and the medical community has been testing for the elusive virus on a sufficiently comprehensive scale.
A new approach to monitoring the novel coronavirus, (as well as other dangerous pathogens and chemical agents), is being developed and refined. Known as wastewater-based epidemiology (WBE), the method mines sewage samples for vital clues about human health. It can potentially identify levels of coronavirus infection at both a local and global scale.Continue reading
LabCorp COVID-19 At-Home Test Kit Receives FDA Emergency Use Authorization
Quest Diagnostics Begins to Perform COVID-19 Antibody Testing
SECAUCUS, N.J., April 21, 2020 /PRNewswire/ — Quest Diagnostics (NYSE: DGX), the world’s leading provider of diagnostic information services, today announced that it has begun to perform antibody testing for coronavirus (COVID-19) using blood samples. With the new service, Quest Diagnostics now provides healthcare providers in the United States access to COVID-19 antibody as well as molecular diagnostic laboratory testing. Continue reading