In June of 2023, the comment period for the proposed rule on Transitional Coverage for Emerging Technologies (TCET) through the national coverage determination (NCD) process under the Social Security Act closed. The draft rule has been released for public inspection and the final rule will be published on August 12, 2024.

Scott Whitaker
AdvaMed President and CEO
Over 150 individuals and organizations, including AZBio, commented on the proposed rule in 2023. (View the comments)
AdvaMed, the Medtech Association, released the following statement from President and CEO Scott Whitaker on the final Transitional Coverage for Emerging Technologies (TCET) notice from the Centers for Medicare and Medicaid Services (CMS):
“The final TCET notice is a step toward a stronger, more robust policy, but doesn’t go far enough to help the Medicare seniors depending on breakthrough diagnostics and treatments to alleviate their suffering. The limited number of devices CMS can handle demonstrates clearly to Congress the need for greater resources at CMS. And the exclusion of diagnostics is disappointing, particularly considering the potential for a breakthrough diagnostic technology to save not only lives but costs to the health care system overall through earlier detection. While we appreciate that CMS has released the rule, we call on Congress to finish the job by passing HR 1691, the Ensuring Access to Critical Breakthrough Products Act. Too many patients in need of breakthrough diagnoses and treatments are counting on it.”
TCET Final Rule
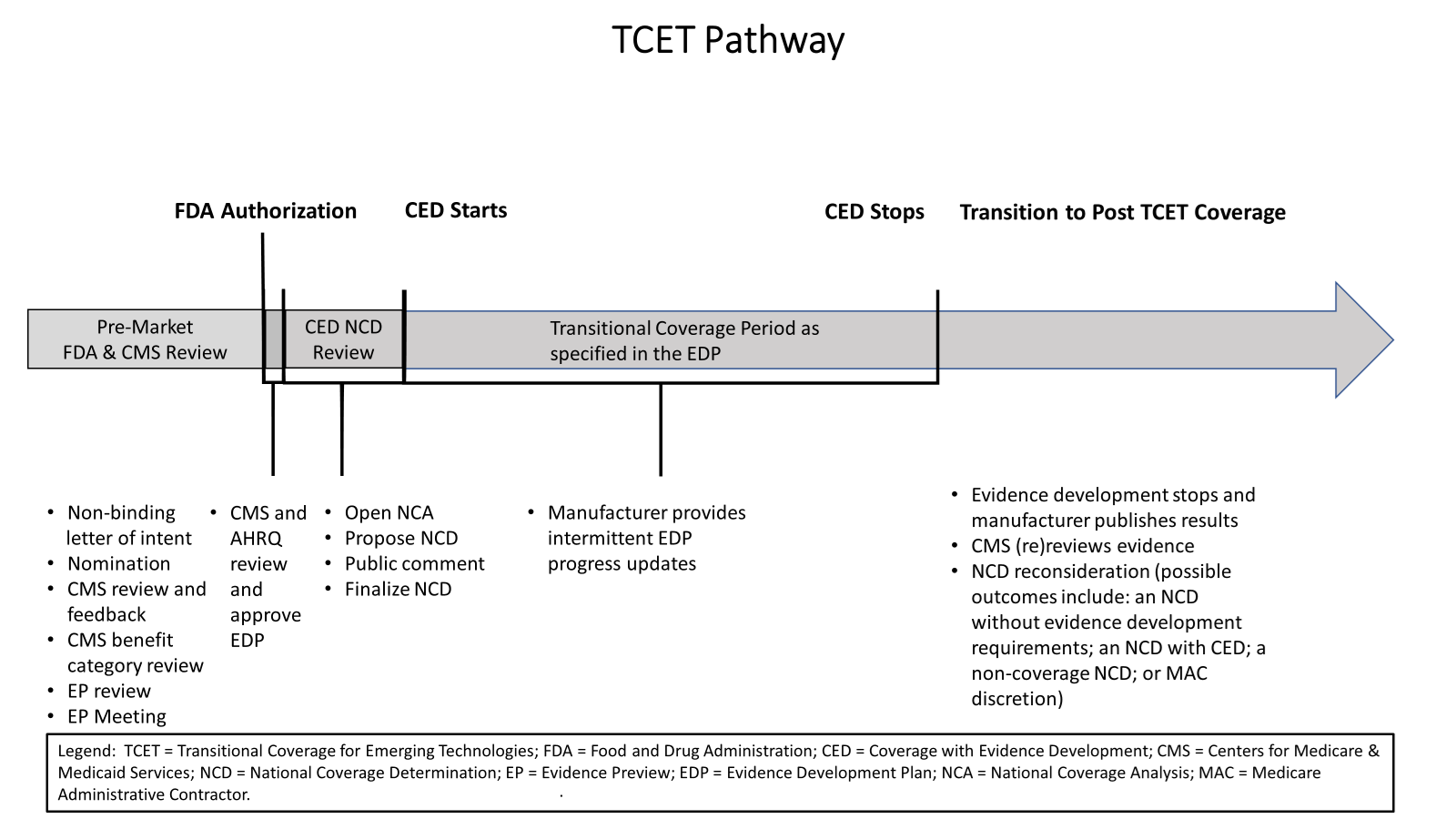
CMS issued a final procedural notice outlining a Medicare coverage pathway to achieve more timely and predictable access to certain new medical technologies for people with Medicare. The new TCET pathway for certain Food & Drug Administration (FDA)-designated Breakthrough Devices increases the number of NCDs that CMS will conduct per year and supports both improved patient care and innovation by providing a clear, transparent, and consistent coverage process while maintaining robust safeguards for the Medicare population. CMS anticipates accepting up to five TCET candidates per year and, for technologies accepted into and continuing in the TCET pathway, CMS’ goal is to finalize a national coverage determination (NCD) within six months after FDA market authorization. View the Public Inspection version of the new TCET Rule. The final rule will be published on August 12, 2024.
TCET Pathway at a Glance
The TCET pathway is intended to balance multiple considerations when making Medicare coverage determinations: (1) facilitating early, predictable, and safer access for people with Medicare to new technologies; (2) reducing uncertainty about coverage by evaluating early the potential benefits and harms of technologies with manufacturers; and (3) encouraging evidence development if notable evidence gaps exist for coverage purposes.