INSYS Therapeutics, Inc. (NASDAQ: INSY), announced om November 1, 2018 that a pharmacokinetic (PK) study (INS012-18-119) of its proprietary intranasal naloxone spray formulations for the treatment of opioid overdose showed a distinctive PK profile compared to the current standards of intramuscular (IM) and intravenous (IV) administration of naloxone.
According to the U.S. Centers for Disease Control and Prevention (CDC), there were 70,689 opioid overdoses in the United States in 2017, of which 28,649 or 40 percent were due to synthetic opioids—a marked increase from 2015, when synthetic opioids accounted for just 18 percent of total opioid overdoses nationwide. As noted by Francis S. Collins, MD, PhD, and Nora Volkow, MD, on the NIH Director’s Blog in May 2017, although naloxone can effectively reverse opioid overdose, its relatively short half-life compared with those of synthetic opioids frequently requires multiple doses to reverse respiratory arrest. The usual doses given of naloxone may not be powerful or long-lasting enough to reverse overdoses from highly potent synthetic opioids.
The INSYS study was a single-dose, open-label, randomized, four-treatment, four-way crossover study. Twenty-four healthy volunteer subjects received one dose each of 8 mg naloxone nasal spray (Formulation I and Formulation II), one dose of 2 mg Naloxone HCl IV Injection (1 mg/mL), and one dose of Naloxone HCl IM Injection (0.4 mg/mL).
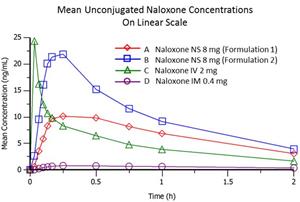
The results from the study, per the graph below, demonstrate that the mean unconjugated naloxone plasma concentrations after administration of the two test nasal formulations:
1) reached appreciably higher levels than those observed after the administration of the IM dose at all timepoints beginning at two minutes; and
2) maintained high levels above the Cmax of 0.4 mg IM for two hours.
Unconjugated naloxone exposure generated from the two test nasal formulations were from 9.6- to 27-fold higher compared to Naloxone 0.4 mg IM.
The results of this study are consistent with the prior study conducted by INSYS (INS012-17-108) that compared the company’s two proprietary nasal formulations with 0.4 mg of Naloxone Intramuscular
“Due to the introduction of synthetic opioids, a more potent, longer-lasting naloxone is required to address an unmet need in today’s opioid epidemic,” said Ahmed Elkashef, M.D., vice president of clinical development at INSYS. “The PK results from this study suggest that our naloxone nasal spray formulation may hold promise to address this unmet need. Based on these results, we look forward to working with the FDA to bring a new treatment to people on the front lines of the opioid crisis.”
Upon receiving the results from the nonclinical juvenile toxicity study, the company plans to file the NDA at the end of the first quarter of 2019.
About INSYS
INSYS Therapeutics is a specialty pharmaceutical company that develops and commercializes innovative drugs and novel drug delivery systems of therapeutic molecules that improve patients’ quality of life. Using proprietary spray technology and capabilities to develop pharmaceutical cannabinoids, INSYS is developing a pipeline of products intended to address unmet medical needs and the clinical shortcomings of existing commercial products. INSYS is committed to developing medications for potentially treating anaphylaxis, epilepsy, Prader-Willi syndrome, opioid addiction and overdose, and other disease areas with a significant unmet need.
SUBSYS® and SYNDROS® are trademarks of INSYS Development Company, Inc., a subsidiary of INSYS Therapeutics, Inc.
NOTE: All trademarks and registered trademarks are the property of their respective owners.
Forward-Looking Statements
This news release contains forward-looking statements including regarding (i) our belief that an intranasal naloxone spray formulation represents a potentially valuable alternative to treat known or suspected opioid intoxication or overdose; and (ii) our intention to launch an intranasal naloxone spray at an affordable price. These forward-looking statements are based on management’s expectations and assumptions as of the date of this news release; actual results may differ materially from those in these forward-looking statements as a result of various factors, many of which are beyond our control. These factors include, but are not limited to, risks described in our filings with the United States Securities and Exchange Commission, including those factors discussed under the caption “Risk Factors” in our Annual Report on Form 10-K for year ended Dec. 31, 2017 and subsequent updates that may occur in our Quarterly Reports on Form 10-Q. Forward-looking statements speak only as of the date of this news release, and we undertake no obligation to publicly update or revise these statements, except as may be required by law.
| CONTACT: | Corporate Communications | Investor Relations |
| Joe McGrath | Jackie Marcus or Chris Hodges | |
| INSYS Therapeutics | Alpha IR Group | |
| 480-500-3101 | 312-445-2870 | |
| jmcgrath@insysrx.com | INSY@alpha-ir.com |
A graph accompanying this announcement is available at http://www.globenewswire.com/NewsRoom/AttachmentNg/e3898b15-e29a-4769-ab49-1a42f0748610
Source: INSYS Therapeutics, Inc.