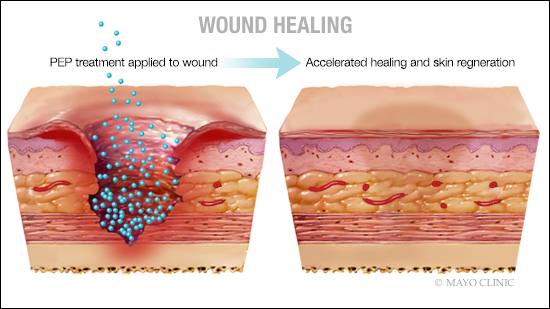
Ischemic wounds occur when arteries are clogged or blocked, preventing important nutrients and oxygen from reaching the skin to drive repair. This groundbreaking study titled, “TGF-β Donor Exosome Accelerates Ischemic Wound Healing,” is published in Theranostics.
Ischemic wounds occur when arteries are clogged or blocked, preventing important nutrients and oxygen from reaching the skin to drive repair. This groundbreaking study titled, “TGF-β Donor Exosome Accelerates Ischemic Wound Healing,” is published in Theranostics.
“This paper documents that PEP, an off-the-shelf, room-temperature-stable exosome, is capable of healing wounds that are depleted of adequate blood supply. Wounds healed with only a single application of exosome,” says Steven Moran, M.D., a Mayo Clinic plastic surgeon and senior co-author on the study. “I was surprised that this product regenerated healthy skin with normal biomechanical properties — not scar tissue. As this technology is now scaled and biomanufactured for clinical applications, it creates the potential for huge advancement in medical science and the field of plastic surgery.”
This study has laid the foundation for Food and Drug Administration approval to begin a first-in-class clinical trial to test safety of using the purified exosomal product for wound healing in patients. This research is supported by Mayo Clinic’s Center for Regenerative Medicine, which is a leader in advancing new, validated regenerative procedures from research into practice.
Chronic ischemic wounds are common in people with conditions such as diabetes, pressure ulcers, hardening of arteries, traumatic injury or side effects of radiation therapy. Standard treatments for these wounds include wound dressing, topical gels and surgery. Although these measures offer some relief, they often cannot fully close the wound. As a result, approximately 7 million people in the U.S. have wounds that don’t close properly, and efforts to find solutions have grown to a multibillion dollar industry, according to National Institutes of Health-sponsored research. When the condition progresses, nonhealing wounds lead to limb amputation.
The purified exosomal product is an extracellular vesicle that delivers cargo from one cell to another, targeting exact tissues in need of repair. This technology is manufactured under strict quality control measures and formulated as a dry powder to enable long-term storage at room temperature. In the operating room or at the bedside, the powder is mixed with a hydrogel solution on-site and can be applied directly to the wound. Unlike cellular products, it does not have to be sent to an outside laboratory to be cultured and scaled.
“What we see with this technology is not just that the wound is closed, but also that the blood supply to the tissue is restored. Our effort culminating in the development of this exosomal technology was to create a therapy that can be offered to all patients in need through elimination of logistical limitations often seen with more traditional regenerative therapy,” says Atta Behfar, M.D., Ph.D., deputy director of Translation, Mayo Clinic’s Center for Regenerative Medicine and senior author. “Our research hopes to answer whether this can be a new healing solution for patients suffering with nonhealing chronic wounds.” Dr. Behfar is director of the Mayo Clinic Van Cleve Cardiac Regenerative Medicine Program where the purified exosomal product was discovered.
The research
The research team replicated wounds with low blood supply in large animal models. They treated some of the wounds with the purified exosomal product and compared them to wounds that were treated with the hydrogel alone. They found wounds treated with the purified exosomal product were able to heal with skin restored to its normal architecture.
“We found that this exosome therapy has the ability to enhance regeneration of blood vessels in damaged tissues. Without treatment, chronic ischemic wounds grow larger and more problematic,” says Ao Shi, Ph.D., a student in the Regenerative Sciences Training Program in Mayo Clinic Graduate School of Biomedical Sciences and first author.
Dr. Behfar is the co-founder of Rion LLC, which Mayo Clinic has licensed to manufacture the purified exosomal product. Mayo Clinic and Dr. Behfar have a financial interest in the technology referenced in this news release.
###
About Mayo Clinic’s Center for Regenerative Medicine
Mayo Clinic’s Center for Regenerative Medicine seeks to integrate, develop and deploy new regenerative medicine products and services that continually differentiate Mayo’s practice to draw patients from around the world for complex care. Learn more on Mayo Clinic’s Center for Regenerative Medicine website.
About Mayo Clinic
Mayo Clinic is a nonprofit organization committed to innovation in clinical practice, education and research, and providing compassion, expertise and answers to everyone who needs healing. Visit the Mayo Clinic News Network for additional Mayo Clinic news. For information on COVID-19, including Mayo Clinic’s Coronavirus Map tracking tool, which has 14-day forecasting on COVID-19 trends, visit the Mayo Clinic COVID-19 Resource Center.
Media contact:
- Susan Buckles, Mayo Clinic Public Affairs, newsbureau@mayo.edu