Metabolic dysregulation in tumors is a topic of intense interest in the study of cancer, and the therapeutic value of altering tumor metabolism is a new frontier in cancer research. Unlike genetic traits that may not be present in all cells of a patient’s tumor, one trait shared by virtually all tumor cells is altered metabolism.
Dr. Adrienne C Scheck and her team at the Barrow Neurological Institute (BNI) have studied the effects of altering metabolism through the use of a ketogenic diet (KD) in combination with radiation and chemotherapy for the treatment of malignant brain tumors. The KD should not be confused with “do-it-yourself” diets found in the popular literature. The diet is a medically administered, highly regimented therapy currently used for the treatment of severe epilepsy in children whose seizures do not respond to drug therapy. It has an excellent safety profile when administered under the guidance of a registered dietitian. It is also thought to have neuroprotective properties, and it is being studied for potential clinical utility in the treatment of Alzheimer’s disease, traumatic brain injury and other neurological disorders.
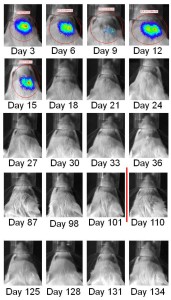
Anecdotal evidence suggests the utility of this therapeutic approach for the treatment of brain tumors, but to date there have been no controlled studies of the use of the KD in combination with the standard of care (radiation and chemotherapy) for primary brain tumors. Scheck’s preclinical data demonstrated enhanced survival when used alone or in combination with chemotherapy. When used in combination with radiation, 9 of 11 mice were cured of their tumors. The Barrow Neurological Institute has now started a Phase I/II clinical trial for patients with newly diagnosed glioblastomas using the ketogenic diet in combination with standard radiation and chemotherapy treatment. Study endpoints include tolerance and compliance with the diet as determined by blood ketone and glucose levels, overall survival, time to recurrence, health and therapy related quality of life.
Phase I/II prospective trial for newly diagnosed GBM, with upfront gross or subtotal total resection, followed by ketogenic diet with radiotherapy and concurrent Temodar® chemotherapy followed by adjuvant Temodar® chemotherapy.