
DuBois, a leader in colorectal cancer research for more than two decades, has been exploring the risk factors between chronic inflammation and cancer. Photo by: Biodesign Institute
A new Arizona State University research study led by Biodesign Institute executive director Raymond DuBois has identified for the first time the details of how inflammation triggers colon cancer cells to spread to other organs, or metastasize.
The findings will enable researchers to identify new drug targets for the prevention and treatment of colon cancer.
DuBois, a leader in colorectal cancer research for more than two decades, has been exploring the risk factors between chronic inflammation and cancer, including colorectal cancer.
“We’ve long known that simple things like taking aspirin or other anti-inflammatory drugs (called nonsteroidal anti-inflammatory drugs, or NSAIDs), have beneficial effects on reducing the risk of colorectal cancer,” DuBois said. “But non-aspirin NSAIDs can cause serious cardiovascular side effects when taken over a long period of time, so we’ve needed to discover better drug targets. This study points us in the right direction.”
The findings could mean new hope for more effective colorectal cancer treatments and screening methods. Currently, nearly half of patients with advanced colorectal cancer die within five years following treatment. One reason may be that the cancer becomes more chemotherapy-resistant, but other evidence has supported the role of inflammation and inflammatory mediators in tumor metastasis.
The research team was able to show how a colon cancer tumor could outwit its host, using an inflammatory mediator to expand its cancer stem cells in order to seek out other organs. They showed a direct link between the pro-inflammatory mediator prostaglandin E2 and increased colorectal cancer stem cells.
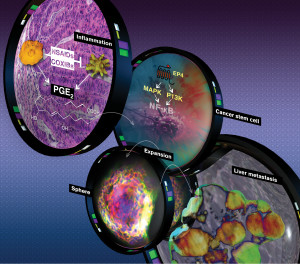
A new Arizona State University research study led by Biodesign Institute executive director Raymond DuBois has identified for the first time the details of how inflammation triggers colon cancer cells to spread to other organs, or metastasize. Photo by: Michael Northrop, Biodesign Institute
A cancer is a genetic menagerie made up of many different types of cells. Some of these cells, called cancer stem cells, are thought to be responsible for tumor initiation, growth, relapse and recurrence.
Prostaglandin E2 is the most abundant pro-inflammatory bioactive fat, or lipid, found in colon, lung, breast, head and neck cancers. When high levels of PGE2 are found in cancer, it often indicates a poor prognosis.
“The normal role of PGE2 is to come to the rescue when you do something like cut your finger,” DuBois said. “It attracts the body’s immune cells and stimulates pathways that heal the wound site. The level of PGE2 goes up and then goes down within a few days of healing the wound.
“But in cancer, the cells keep making PGE2 chronically, so it’s like this wounding process that never heals. In doing so, it generates these cancer stem cells that promote cancer progression and metastatic spread.”
DuBois’ research team proved that when PGE2 binds to its receptor on the surface of the cancer cell, PGE receptor 4, or EP4, it triggers a signaling cascade for cancer stem cells to renew, differentiate and eventually become resistant to chemotherapy.
It’s as if the original cancer tumor cells, starved for oxygen and running low on supplies in the colon, trick its host body by making PGE2 to send out a help signal for the new stem cells to build a life raft and find safe harbor throughout the body and multiply again.
DuBois was able to show in a mouse model of colorectal cancer that adding PGE2 increased the number of cancer stem cells (CSCs) and overall tumor burden, with the liver being one of the prime organ targets for colorectal cancer to first spread.
“We use a combination of approaches that utilize human tissues and mouse models to complete our studies,” DuBois said. “We take samples from human colon cancers and then purify the cancer stem cell population. When we treat these stem cells with PGE2, we found that they are 1,000 times more metastatic than the cells we don’t treat with PGE2.”
In addition, the elevated PGE2 levels and increased numbers of CSCs were also seen in human colon cancer tissue specimens.
Furthermore, when they were able to block the PGE2 signaling by adding a drug that inhibits binding to the EP4 receptor, it reduced the number of polyps and CSCs, and blocked liver metastasis.
The paper was the first to show the impact PGE2 had on the spread of CSCs to the liver. With the new proof in hand, DuBois is optimistic about future research.
“Now, if we can just target and eliminate the stem cells from people with colon cancer, we can develop a new therapeutic approach to treat colorectal cancer and improve outcomes,” DuBois said.
DuBois is hopeful that human clinical trials can be initiated in a reasonable time to show the effectiveness of the new drug target strategy.
The findings appear in the latest issue of the journal Gastroenterology. Key contributors from DuBois’ Laboratory for Cancer and Inflammation at Biodesign include Dingzhi Wang, Lingchen Fu and Haiyan Sun; and Lixia Guo, from Mayo Clinic, Rochester. The study was supported by grants from the National Institutes of Health and the National Colorectal Cancer Research Alliance.
