Already considered a global epidemic, human obesity continues to be on the rise. According to the Centers for Disease Control, more than 40% of the U.S. population is considered obese. The gamut of adverse health effects associated with obesity is broad, including such devastating illnesses as type 2 diabetes, coronary artery disease, stroke, sleep apnea and certain forms of cancer. Patients often suffer depression, loss of mobility, social isolation and inability to work.
With costs approaching $316 billion dollars annually in the U.S., understanding how to quell obesity will result not only in a healthier population, but could also help reduce runaway medical costs.
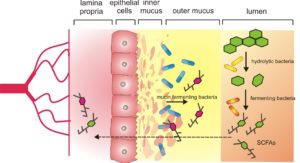
Figure from “Microbiome After Bariatric Surgery and Microbial Insights into Surgical Weight Loss, A Dissertation Presented in Partial Fulfillment of the Requirements for the Degree: Doctor of Philosophy” by Esra Zehra Ilhan
Despite the looming need to address obesity, the causes are not well understood. Researchers generally agree that genetic and gut microbiome composition and activity are important factors in determining who is obese and who isn’t.
As interest and understanding of the human microbiome increases, researchers are increasingly looking to the gut for answers that can lead to new, more effective diagnostics and therapies.
The trillions of microbes in the human gut perform a vast range of critical functions in the body and have even been implicated in mood and behavior. Among microbes’ critical responsibilities are the micro-management of nutrients in the food we digest – one of the reasons for their central role in the regulation of body weight.
In a new study, “Temporospatial shifts in the human gut microbiome and metabolome after gastric bypass surgery,” recently published in npj Biofilms and Microbiomes, ASU researcher Zehra Esra Ilhan, ASU Biodesign professor Rosa Krajmalnik-Brown—and researchers from Mayo Clinic and Pacific Northwest National Laboratory, have taken another step in understanding how the gut changes after gastric bypass surgery (also known as Roux-en-Y gastric bypass surgery).
“Our findings highlight the importance of changes in mucosal and fecal microbiomes that are reflected on gut metabolism after surgery,” said Ilhan. The microbial changes after surgery corresponded to persistent changes in fecal fermentation and bile acid metabolism, both of which are associated with improved metabolic outcomes.”
In addition to the expected weight reduction and improvement in obesity-related comorbidities after gastric bypass surgery, the researchers observed that the impact of surgery is not limited to fecal communities; mucosal communities are altered as well. Changes in the microbiome were linked to increased concentrations of branched-chain fatty acids (amino acid fermentation products) and an overall decrease in primary and secondary bile acid concentrations in fecal samples. Bile is an alkaline fluid that aids digestion.
“Previous bariatric surgery-microbiome studies in humans relied largely on fecal samples because sampling through the intestinal mucosal membrane requires an invasive procedure,” said Ilhan, lead researcher for the study. At the time of the study, Ilhan was a doctoral student in Krajmalnik-Brown’s lab. She is currently conducting research at INRAE-French National Institute for Agriculture, Food and Environment.
Bariatric surgery is an operation that causes people to lose weight by making changes to the digestive system. These changes are physiological and chemical and include gastric restriction, malabsorption, bile acid metabolism, and chemical signaling.
“The mucus membrane is a critically important site for host-microbe interactions. We understood that with fecal sampling, we had an underrepresented picture of how the mucosal communities actively interact with host immune system and epithelial cells,” said Ilhan. (Epithelial cells are cells that line the surfaces of your body.)
Although gastric bypass surgery has been successful for many patients suffering from morbid obesity, it is a serious, invasive procedure that is not without risk and expense. In addition, some patients regain the weight they have lost, perhaps because they lack the favorable microbes necessary for permanent weight loss.
“Understanding the microbial behavior in the gut could potentially lead to a creating a probiotic that could replace surgery – or an improved indicator to identify the best candidates for surgery and sustained weight loss,” said Krajmalnik-Brown.
In the longitudinal study, subjects provided fecal samples and rectal mucosal samples. The rectal mucosal samples were collected via un-sedated flexible sigmoidoscopy at baseline, and again at 12 months post-gastric bypass surgery. Researchers analyzed microbial DNA that was extracted from the fecal and mucosal samples. Fecal metabolites were analyzed using high-throughput metabolomics approaches.
A tell-tale indicator of pathology in obese patients has been found in the gut, where a markedly lower diversity of microbial communities is observed. A high diversity of gut microbes is essential to good health.
In 2009, Krajmalnik-Brown’s research team showed for the first time that gastric bypass surgery produced profound changes in the composition of microbial communities in the gut. The gut flora of post-surgical gastric bypass patients showed a marked difference from obese and normal weight patients.
In a 2017 study, the team took another step by comparing how microbes and the metabolome (the metabolome is the total number of metabolites present within an organism, cell or tissue) change after gastric bypass surgery and lap band surgery.
The 2017 study demonstrated that gastric bypass surgery caused a dramatic reorganization of the gut, which increases microbial diversity. Changes in the gut microbiota related to lap band surgery (also known as laparoscopic adjustable gastric banding) were mild and accompanying weight loss was less pronounced. Earlier studies have demonstrated that fat is reduced and weight loss triggered when germ-free mice receive a fecal transplant from mice who had undergone gastric bypass surgery.
Krajmalnik-Brown’s team is currently working on a project funded by the National Institutes of Health in which the main goal is to quantify the contribution of the microbiome to the host energy balance. This project is intended to move the field from associations to causality and help identify how microbes and metabolites can fight obesity.
Krajmalnik-Brown is a professor in the ASU School of Sustainable Engineering and Built Environment and faculty in the School of Life Sciences. At the Biodesign Institute, she practices as part of the Swette Center for Environmental Biotechnology.
Krajmalnik-Brown is also known for her research into the role of the microbiome in those with autistic spectrum disorder.
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01DK090379
By Dianne Price, ASU Biodesign Institute
